Second law of thermodynamic puts a fundamental limit on the working performance of a heat engine or a refrigerator. Isolated systems spontaneously evolve towards thermal equilibriumthe state of maximum entropy of the system.

3 Second Law Of Thermodynamics And Entropy Flashcards Quizlet
What is the second law of thermodynamics.
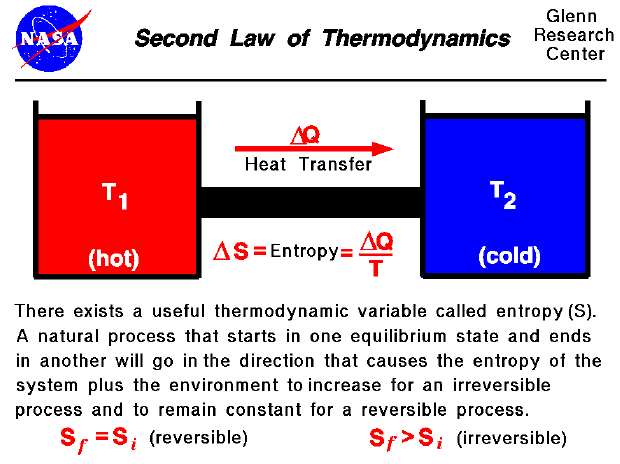
. The second law of thermodynamics states that the total entropy of an isolated system can never decrease over time and is constant if and only if all processes are reversible. 3rd Law of Thermodynamics - A perfect crystal at zero Kelvin has zero entropy. The Thermal Bottleneck Second Law of Thermodynamics says you cant even break even.
5 What does the 2nd law of thermodynamics say and how does it apply to living things. Entropy is a measure of the randomness of the system or it is the measure of energy or chaos within an isolated system. 3 What does the second law of thermodynamics have to do with.
2nd Law of Thermodynamics - For a spontaneous process the entropy of the universe increases. 2 How does the second law of thermodynamics allow for diffusion of substances quizlet. Mathematically the second law of thermodynamics is represented as.
Application of the second law of thermodynamics helps explain the various ways in which engines transform heat into mechanical work as for instance in the gasoline engine of a car or in a steam turbine. The second law of thermodynamics postulates that the entropy of a closed system will always increase with time and never be a negative value. But the Second Law says that no heat engine can use all the heat produced by a fuel to do work.
Maximum efficiency is achieved by a thermodynamic cycle which is a reversible cycle called Carnot cycle. The entropy of the universe the ultimate isolated system only increases and never decreases. 1st Law of Thermodynamics - Energy cannot be created or destroyed.
This is also commonly referred to as entropy. Where ΔS univ is the change in the entropy of the universe. As the universe runs down order is converted to random disorder which is.
4 How does the second law apply to movement of molecules across membranes. The second law of thermodynamics states that the total entropy of an isolated system can never decrease over time and is constant if and only if all processes are reversible. Human organisms are not a closed system and thus the energy input and output of an the organism is not relevant to the second law of thermodynamics directly.
Second Law of Thermodynamics Equation. Isolated systems spontaneously evolve towards thermodynamic equilibrium the state with maximum entropy. The Carnot cycle sets the ideal efficiency which can be obtained if there is no friction mechanical losses leakage etc but real machine efficiencies are much less.
6 How does diffusion increase entropy. ΔS univ 0. Whenever energy is changed in form some of it is changed to heat.
What are the uses of the Second Law of Thermodynamics. The second law of thermodynamics says that the entropy of any isolated system always increases. The Second Law of Thermodynamics states that in all energy exchanges if no energy enters or leaves the system the potential energy of the state will always be less than that of the initial state.
The coefficient of performance of a refrigerator can never be infinite. Isolated systems spontaneously evolve towards thermodynamic equilibrium the state with maximum entropy.

Thermodynamics Vocab Flashcards Quizlet
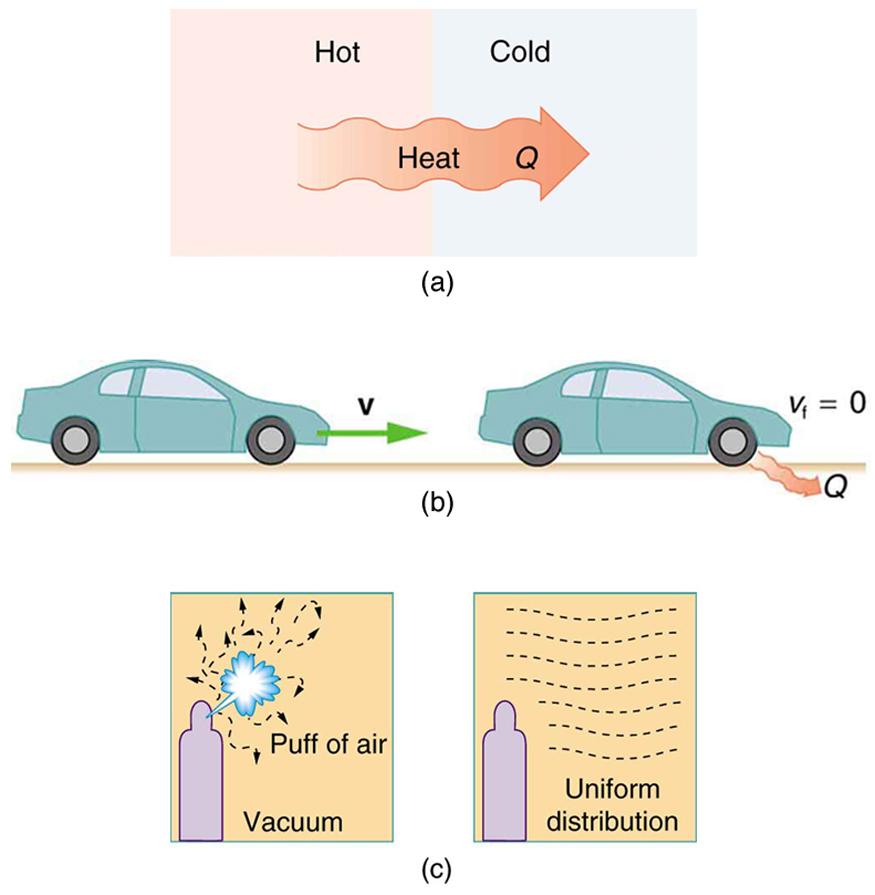
Introduction To The Second Law Of Thermodynamics Heat Engines And Their Efficiency College Physics
0 Comments